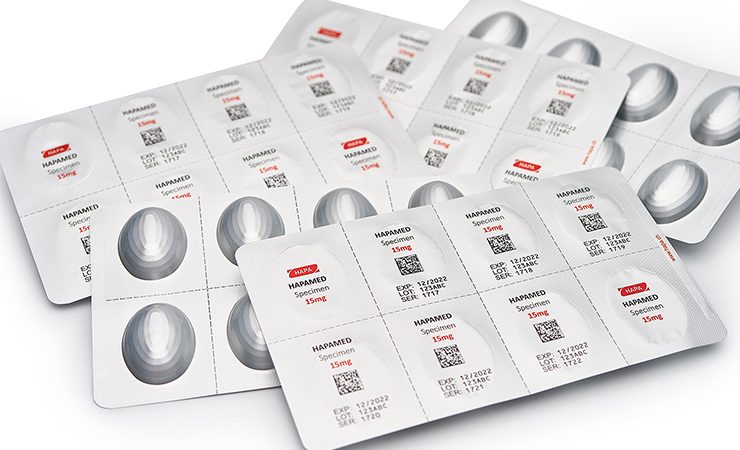
Hapa has introduced new drop-on-demand (DoD) UV inkjet digital printing technology for the pharma market, with VDP 827 an inline system that has been designed to make marking and coding more efficient and reliable when producing pharmaceutical blister packs.
Hapa noted that, despite having long set up times, poor legibility and rework implications, mechanical embossing remains the dominant technology for adding variable data on blister packs. UV DoD technology on the other hand is noted to offer multiple benefits to manufacturers, such as enhanced machine readability and minimised waste.
Based on Hapa’s Webjet technology, VDP 827 seeks to change this by affording inline DoD marking and coding at a resolution of 360dpi, print across the full web width up to 288mm, serialisation or coding on any part of the blister foil, and via a compact unit integrated inline into blister lines. To further underline this capability, the VDP concept has been developed in consultation with the leading blister machine manufacturers. VDP 8257 also supports all standard symbologies and barcodes, and does not generate any dust or dirt unlike ablative laser systems. Foils do not need to be coated prior to printing when using the system, so reducing upfront material costs.
Hapa sales director James MacKenzie explained, ‘To achieve this level of standardisation, we worked closely together with several manufacturers at the development stage. Pharmaceutical companies thus attain printing processes that are optimally matched to the blister machines and benefit from standardized spare and wear parts that are available worldwide.’
He added, ‘VDP 827 offers a simple and affordable pathway to the rewards of probably the most advanced inline printing technology for this particular application. It improves patient safety, reduces production complexity, cuts cost and increases OEE.’
Read more about the pharmaceutical market in the July/August 2021 issue of Digital Labels & Packaging; register here to receive the magazine, for free